FDA grants priority review to Tukysa regimen for advanced colorectal cancer
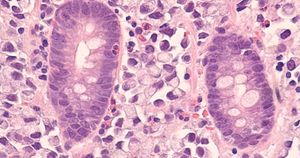
The FDA granted priority review to tucatinib in combination with trastuzumab for treatment of certain adults with colorectal cancer.The designation applies to use of the agent by patients who received at least one prior treatment for unresectable or metastatic disease.The agency is expected to make a decision on approval by Jan. 19.Tucatinib (Tukysa, Seagen) is an oral tyrosine kinase inhibitor that is highly selective for HER2 without significant inhibition of EGFR. The agent is approved in the United States in combination with trastuzumab (Herceptin, Genentech) and capecitabine forRead More